-
This is a political forum that is non-biased/non-partisan and treats every person's position on topics equally. This debate forum is not aligned to any political party. In today's politics, many ideas are split between and even within all the political parties. Often we find ourselves agreeing on one platform but some topics break our mold. We are here to discuss them in a civil political debate. If this is your first visit to our political forums, be sure to check out the RULES. Registering for debate politics is necessary before posting. Register today to participate - it's free!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Today in science and space
- Thread starter HangLow
- Start date
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
The Kola borehole is still the record holder for "true vertical depth" and is a testament to the Soviet urge to break records for no scientific or economic purpose.
"Moon, kshmoon, we will beat you to the center of the Earth itself!"
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
Outstanding...
I was surprised that I had heard of so many of the sites...
But never viewed them from a comparative depth perception...
Very Kool... Thanks...
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
Do you know why they stopped drilling?The Kola borehole is still the record holder for "true vertical depth" and is a testament to the Soviet urge to break records for no scientific or economic purpose.
"Moon, kshmoon, we will beat you to the center of the Earth itself!"
I understand that the stoppage was abrupt and no reason was given...
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
Do you know why they stopped drilling?
I understand that the stoppage was abrupt and no reason was given...
I think the money stopped. They were privatized after the fall of the Soviets, and tried to run it as a tourist attraction. But I guess there isn't much to see with a borehole.
The fact they had to branch off repeatedly from the main (vertical) bore suggests they were having trouble with the rock being too hot and plastic to drill. If your purpose was to actually reach the mantle, you'd dig on an oceanic plate instead: it's a lot thinner and the seawater above cools it more effectively.
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
After watching that video, I tried to work out what air pressure would be in a deep mine or subway. But I got a ridiculous answer. Perhaps someone can set me straight:
Ph = P0 x e^(-mgh/kT)
where
P0 is sea level pressure, 1 atm
m is mass per air molecule, 28 Da = 4.65 x 10^-23 g
g is force of gravity, very close to 9.81 m/s^2
h is -3000 m
k is the Boltzman constant, 1.38 x 10^-23 J/K
T is absolute temperature, say 25 C = 298 K
The calculation is fairly basic, since the tiny exponents cancel out. But having h be negative kicks out the power of e massively. Given that the air column gets denser and denser the lower you go, and the mass of that air column increases cumulatively with the pressure, I was ready for anything up to 10 atmospheres at 3 kilometers down.
What I actually got: 2.53 x 10^144 atm
Oops!
Ph = P0 x e^(-mgh/kT)
where
P0 is sea level pressure, 1 atm
m is mass per air molecule, 28 Da = 4.65 x 10^-23 g
g is force of gravity, very close to 9.81 m/s^2
h is -3000 m
k is the Boltzman constant, 1.38 x 10^-23 J/K
T is absolute temperature, say 25 C = 298 K
The calculation is fairly basic, since the tiny exponents cancel out. But having h be negative kicks out the power of e massively. Given that the air column gets denser and denser the lower you go, and the mass of that air column increases cumulatively with the pressure, I was ready for anything up to 10 atmospheres at 3 kilometers down.
What I actually got: 2.53 x 10^144 atm
Oops!
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
hey @Gatsby and @Glitch; see above post...
do you two brainiacs want to give @Ug make hammer a hand ^^^^
do you two brainiacs want to give @Ug make hammer a hand ^^^^
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
hey @Gatsby and @Glitch; see above post...
do you two brainiacs want to give @Ug make hammer a hand ^^^^
I'm guessing the short answer is that I have the wrong formula. Or it's just an approximation which doesn't work for negative h.
It would be good to know though. Ten to the power of 144 is not just a little bit wrong!
- Joined
- Apr 3, 2019
- Messages
- 22,341
- Reaction score
- 9,893
- Location
- Alaska (61.5°N, -149°W)
- Gender
- Male
- Political Leaning
- Conservative
To calculate the atmospheric pressure for a given altitude use:After watching that video, I tried to work out what air pressure would be in a deep mine or subway. But I got a ridiculous answer. Perhaps someone can set me straight:
Ph = P0 x e^(-mgh/kT)
where
P0 is sea level pressure, 1 atm
m is mass per air molecule, 28 Da = 4.65 x 10^-23 g
g is force of gravity, very close to 9.81 m/s^2
h is -3000 m
k is the Boltzman constant, 1.38 x 10^-23 J/K
T is absolute temperature, say 25 C = 298 K
The calculation is fairly basic, since the tiny exponents cancel out. But having h be negative kicks out the power of e massively. Given that the air column gets denser and denser the lower you go, and the mass of that air column increases cumulatively with the pressure, I was ready for anything up to 10 atmospheres at 3 kilometers down.
What I actually got: 2.53 x 10^144 atm
Oops!
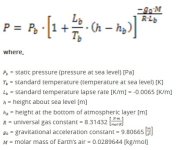
At a depth of 3 km, using a temperature of 19.5°C (-3,000 x -0.0065), the atmospheric pressure should be 1.40 atm (141.855 kPa or 20.57 psi).
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
To calculate the atmospheric pressure for a given altitude use:
View attachment 67429243
At a depth of 3 km, using a temperature of 19.5°C (-3,000 x -0.0065), the atmospheric pressure should be 1.40 atm (141.855 kPa or 20.57 psi).
Most of the same variables and constants, but without that deadly power of e. Your result is a bit under what I would expect, but it's not crazy like my result. I'll go with that, thanks!
- Joined
- Apr 3, 2019
- Messages
- 22,341
- Reaction score
- 9,893
- Location
- Alaska (61.5°N, -149°W)
- Gender
- Male
- Political Leaning
- Conservative
The reason it appears so low is because we are talking about the atmosphere, and not a liquid. In sea water or even fresh water, the pressure is going to be considerably greater at that depth. In sea water the atmospheric pressure at 3 km depth would be 300.165 atm (4,411.209 psi). Freshwater is less dense, therefore at 3 km depth the atmospheric pressure would be 291.451 atm (4,283.156 psi). It also gets colder at depth in water, whereas it gets warmer at depth when not in water. For example, the Kola Super Deep Bore Hole at a depth of 12.272 km would have a temperature that is 79.768°C above the temperature at sea level, even though it would only have an atmospheric pressure of 2.4 atm (35.27 psi) at that depth.Most of the same variables and constants, but without that deadly power of e. Your result is a bit under what I would expect, but it's not crazy like my result. I'll go with that, thanks!
There is never a good reason to use the Stefan-Boltzmann constant. It is based upon a theoretical object that cannot exist in the real-world, a black body, and therefore should only be used to provide very rough estimates and never relied on for accurate information.
Last edited:
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
Latest News
- Joined
- Jan 29, 2012
- Messages
- 2,607
- Reaction score
- 531
- Gender
- Male
- Political Leaning
- Other
And now for something completely different:
Molten rock solidifying in a vacuum under 1/6th gravity is different from the Earth varieties.
Shocking!
Molten rock solidifying in a vacuum under 1/6th gravity is different from the Earth varieties.
Shocking!
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
Here's a kicker; Seawater and Moondust forever...And now for something completely different:
Molten rock solidifying in a vacuum under 1/6th gravity is different from the Earth varieties.
Shocking!
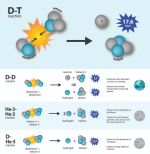
Much speculation has been created around the idea of harvesting Helium-3 from the moon to use as a clean energy source. The promise of commercial fusion power has fuelled what was once thought of as science fiction. So, how close are we to starting mining lunar Helium-3?
Unsurprisingly, the aspiration of mining Helium-3 from the Moon has attracted much interest recently. A study from the University of Wisconsin reports that about 25 tons of Helium-3, corresponding to a fully-loaded spaceship, could power the United States for a year.
In an economic point of view, this means one ton of Helium-3 would cost $3 billion (can you think of other commodities with a similar price?). So, why are we not mining the Moon yet?
One stumbling block is designing a spacecraft to carry the equipment and crew to the lunar surface. Despite being created 40 years ago, the Apollo Saturn V remains the largest spaceship to date with a capacity of 50 tons. Even if it is still the benchmark of a reliable space rocket, more advanced space technology may be required to allow extraterrestrial mining.
Another issue is the mining process itself. To extract Helium-3, the surface of the Moon needs to be heated to 600° C (1,112° F). However, because the concentrations are low, over 150 tons of lunar regolith are required to obtain one gram of Helium-3. Despite not being a particularly difficult extraction process, the development of the machinery, transportation, and infrastructure has a total estimated cost of ca. $20 billion.

Helium-3 Mining On The Moon: Houston, We Have A Problem | Geology for Investors
www.geologyforinvestors.com
Also:

China discovers new mineral – and a possible energy source – on the Moon
It seems China's Chang'E-5 robotic Moon mission has discovered more than water on the lunar surface. Scientists have confirmed the discovery of a new mineral, a transparent crystal named Changesite-(Y), as well as a promising potential fusion fuel.
Last edited:
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
Here's a kicker; Seawater and Moondust forever...
Much speculation has been created around the idea of harvesting Helium-3 from the moon to use as a clean energy source. The promise of commercial fusion power has fuelled what was once thought of as science fiction. So, how close are we to starting mining lunar Helium-3?
Unsurprisingly, the aspiration of mining Helium-3 from the Moon has attracted much interest recently. A study from the University of Wisconsin reports that about 25 tons of Helium-3, corresponding to a fully-loaded spaceship, could power the United States for a year.
In an economic point of view, this means one ton of Helium-3 would cost $3 billion (can you think of other commodities with a similar price?). So, why are we not mining the Moon yet?
One stumbling block is designing a spacecraft to carry the equipment and crew to the lunar surface. Despite being created 40 years ago, the Apollo Saturn V remains the largest spaceship to date with a capacity of 50 tons. Even if it is still the benchmark of a reliable space rocket, more advanced space technology may be required to allow extraterrestrial mining.
Another issue is the mining process itself. To extract Helium-3, the surface of the Moon needs to be heated to 600° C (1,112° F). However, because the concentrations are low, over 150 tons of lunar regolith are required to obtain one gram of Helium-3. Despite not being a particularly difficult extraction process, the development of the machinery, transportation, and infrastructure has a total estimated cost of ca. $20 billion.

Helium-3 Mining On The Moon: Houston, We Have A Problem | Geology for Investors
www.geologyforinvestors.com
Also:

China discovers new mineral – and a possible energy source – on the Moon
It seems China's Chang'E-5 robotic Moon mission has discovered more than water on the lunar surface. Scientists have confirmed the discovery of a new mineral, a transparent crystal named Changesite-(Y), as well as a promising potential fusion fuel.newatlas.com
Deuterium/Tritium requires a higher activation energy than D/D. It's selling point is that it doesn't emit neutrons (which are quite frankly nuclear waste) but we don't have stable D/D fusion yet. "Twenty years away" as they always say.
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
Deuterium/Tritium requires a higher activation energy than D/D. It's selling point is that it doesn't emit neutrons (which are quite frankly nuclear waste) but we don't have stable D/D fusion yet. "Twenty years away" as they always say.
Oops, I meant Helium-3 not Tritium. Deuterium/Tritium still releases neutrons.
And for all we know, Helium-3/Helium-3 might too. Nuclear reactions are probabilistic, there's always a side chain. We could find out how much of a problem nuclear waste from He-3 actually is, using the NIF, long before harvesting it from the Moon.
- Joined
- Jan 29, 2012
- Messages
- 2,607
- Reaction score
- 531
- Gender
- Male
- Political Leaning
- Other
That's alright I didn't notice. I have been hearing that fusion power is 20 years away for so long I don't even pay attention.Oops, I meant Helium-3 not Tritium. Deuterium/Tritium still releases neutrons.
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
less than 20 years...That's alright I didn't notice. I have been hearing that fusion power is 20 years away for so long I don't even pay attention.
five to ten...
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
- Joined
- Aug 19, 2014
- Messages
- 42,333
- Reaction score
- 31,593
- Location
- Tennessee, USA
- Gender
- Male
- Political Leaning
- Conservative
Heads up! A retired NASA ozone layer-studying satellite from 1984 is comin' down!


Fiery Destruction Expected: NASA Earth Radiation Budget Satellite To Reenter Atmosphere Today
In early January NASA’s retired Earth Radiation Budget Satellite (ERBS) is expected to reenter Earth’s atmosphere after almost four decades in space. For 21 of those years, the ERBS actively investigated how the Earth absorbed and radiated energy from the Sun, and made measurements of stratospheric
scitechdaily.com
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
- Joined
- Feb 22, 2019
- Messages
- 37,166
- Reaction score
- 24,119
- Location
- The Bay
- Gender
- Male
- Political Leaning
- Progressive
Not forever.Love it though it's not my brainchild, just a great thread to have
The article about tracing the origins of the Black Plague is particularly interesting. I didn't know DNA of plagues survive in teeth.
- Joined
- Sep 11, 2021
- Messages
- 18,826
- Reaction score
- 11,576
- Gender
- Undisclosed
- Political Leaning
- Very Liberal
| A type of freshwater plankton has become the first organism seen thriving on a diet of viruses, according to a new study by researchers from the University of Nebraska-Lincoln in the US. |
"And then I see the plankton where it knocks it out in a minute. One minute. And is there a way we can do something like that, by injection inside or almost a cleaning?"
You first Donald.
- Joined
- Sep 27, 2020
- Messages
- 21,382
- Reaction score
- 17,727
- Gender
- Male
- Political Leaning
- Other
I thought of that... COVID-eating plankton...
A type of freshwater plankton has become the first organism seen thriving on a diet of viruses, according to a new study by researchers from the University of Nebraska-Lincoln in the US.
"And then I see the plankton where it knocks it out in a minute. One minute.
And is there a way we can do something like that, by injection inside or almost a cleaning?"
You first Donald.
either that or stick a U-V lite up his butt...
it may just work... the bastard was a genius...
Last edited: