Hypothetical alternatives to water include ammonia, which, like water, is a polar molecule, and cosmically abundant; and non-polar hydrocarbon solvents such as methane and ethane, which are known to exist in liquid form on the surface of Titan.
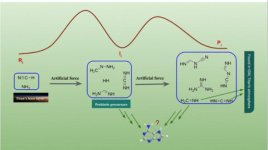
| Ammonia biochemistry | Non-water solvents | Ammonia-based life | Ammonia is relatively abundant in the universe and has chemical similarities to water. The possible role of liquid ammonia as an alternative solvent for life is an idea that goes back at least to 1954, when J. B. S. Haldane raised the topic at a symposium about life's origin. |
In addition to carbon compounds, all currently known terrestrial life also requires water as a solvent. This has led to discussions about whether water is the only liquid capable of filling that role. The idea that an extraterrestrial life-form might be based on a solvent other than water has been taken seriously in recent scientific literature by the biochemist
Steven Benner and by the astrobiological committee chaired by John A. Baross.
[41] Solvents discussed by the Baross committee include
ammonia...
==============================================================
Ammonia
The
ammonia molecule (NH3), like the water molecule, is abundant in the universe, being a compound of hydrogen (the simplest and most common element) with another very common element, nitrogen.The possible role of liquid ammonia as an alternative solvent for life is an idea that goes back at least to 1954, when
J. B. S. Haldane raised the topic at a symposium about life's origin.
However, ammonia has some problems as a basis for life. The
hydrogen bonds between ammonia molecules are weaker than those in water, causing ammonia's
heat of vaporization to be half that of water, its
surface tension to be a third, and reducing its ability to concentrate non-polar molecules through a
hydrophobic effect. Gerald Feinberg and Robert Shapiro have questioned whether ammonia could hold prebiotic molecules together well enough to allow the emergence of a self-reproducing system. Ammonia is also flammable in oxygen and could not exist sustainably in an environment suitable for
aerobic metabolism.
A
biosphere based on ammonia would likely exist at temperatures or air pressures that are extremely unusual in relation to life on Earth. Life on Earth usually exists within the melting point and
boiling point of water, at a pressure designated as
normal pressure, and between 0 and 100 °C (273 and 373
K). When also held to normal pressure, ammonia's melting and boiling points are −78 °C (195 K) and −33 °C (240 K) respectively. Because chemical reactions generally proceed more slowly at lower temperatures, ammonia-based life existing in this set of conditions might metabolize more slowly and evolve more slowly than life on Earth. On the other hand, lower temperatures could also enable living systems to use chemical species that would be too unstable at Earth temperatures to be useful.