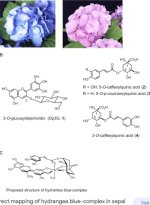
It's been far too long since I took chemistry....
An awful lot of google results agree that epsom salts
are usually PH neutral because of what happens during uptake, but also agree that excessively low (or high) PH can reduce magnesium bioavailability.
Epsom salt is used by many growers as a fertilizer supplement to provide magnesium and sulfate when they are low in the fertilizer solution

www.pthorticulture.com
Epsom salts contain sulfur and magnesium, and you can add them to the soil when it's...

homeguides.sfgate.com
Used by many gardeners as a supplemental fertilizer for vegetables and ornamentals, Epsom...

homeguides.sfgate.com
Three common "remedies" may or may not be helpful in the garden.

extension.umn.edu
At any rate, OP seems to have done a good amount of guesswork in identifying magnesium depletion as the cause of less flowering. What about average rainfall in various months? Temperature? Possible environmental contaminents (did a neighbor use a broadleaf killer that washed onto your property?)
That said....
.....that's for
lowering PH! Now it makes sense. Epsom Salts aren't affecting your soil PH, that aluminum salt
is, and once you knock PH too low you easily get iron and magnesium deficiencies. Adding more magnesium isn't going to help. It won't get uptaken. (
In fact, having too much magnesium will give you a second problem: inhibited calcium uptake.
Guess what else isn't good: too much iron. So you may end up causing either two or three problems when you had one with the epsom salt switchover plan.
Test that soil PH.....that was good advice. Like, now.